Journal Description
Clinics and Practice
Clinics and Practice
is an international, scientific, peer-reviewed, open access journal on clinical medicine, published bimonthly online by MDPI (from Volume 11, Issue 1 - 2021).
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, ESCI (Web of Science), PubMed, PMC, Embase, and other databases.
- Journal Rank: CiteScore - Q2 (General Medicine)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 26.4 days after submission; acceptance to publication is undertaken in 3.8 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
Impact Factor:
2.3 (2022);
5-Year Impact Factor:
2.0 (2022)
Latest Articles
Giant Intraabdominal Lymphangioma in a Pediatric Patient—A Challenging Diagnosis
Clin. Pract. 2024, 14(3), 739-748; https://doi.org/10.3390/clinpract14030059 (registering DOI) - 25 Apr 2024
Abstract
►
Show Figures
Introduction: Intra-abdominal cystic formations represent heterogeneous pathologies with varied localization and clinical manifestation. The first challenge of a giant intra-abdominal cystic lesion is identifying the organ of origin. The clinical presentation of intra-abdominal cystic lesions varies from acute manifestations to non-specific symptoms
[...] Read more.
Introduction: Intra-abdominal cystic formations represent heterogeneous pathologies with varied localization and clinical manifestation. The first challenge of a giant intra-abdominal cystic lesion is identifying the organ of origin. The clinical presentation of intra-abdominal cystic lesions varies from acute manifestations to non-specific symptoms or accidental discovery. Case presentation: A 2-year-old girl presents to the emergency unit with a fever of 38.5 Celsius, loss of appetite, and apathy. The investigations showed a gigantic intra-abdominal mass whose organ belonging could not be specified. Postoperatively, a giant mesenteric lymphangioma was evident, which was completely excised. Discussion: Giant cystic formations modify the anatomical reports and become space-replacing formations, and the starting point is even more challenging to assess preoperatively. Nevertheless, the careful evaluation of the characteristics of the formation, the effect on the adjacent organs, the age of the patient, and the clinical picture can provide elements of differential diagnosis. The stated purpose of this work is to systematize intra-abdominal lesions according to the organ of origin and to make the preoperative diagnosis of an intra-abdominal cystic lesion in the pediatric patient easy to perform starting from the presented case.
Full article
Open AccessArticle
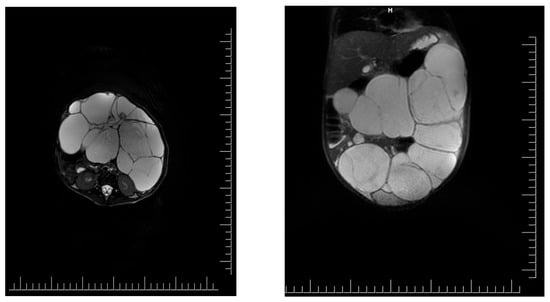
Public Perceptions of Rotator Cuff Tears
by
V. V. N. Manohar Devarasetty, John E. Kuhn and Eric N. Bowman
Clin. Pract. 2024, 14(3), 729-738; https://doi.org/10.3390/clinpract14030058 (registering DOI) - 25 Apr 2024
Abstract
(1) Background: Full-thickness rotator cuff tears (RCTs) impact 25% of those over 60 and 50% over 80; however, minimal data exists on public understanding; (2) Methods: The primary outcome was to determine the public’s baseline understanding of RCTs utilizing a 36-question survey regarding
[...] Read more.
(1) Background: Full-thickness rotator cuff tears (RCTs) impact 25% of those over 60 and 50% over 80; however, minimal data exists on public understanding; (2) Methods: The primary outcome was to determine the public’s baseline understanding of RCTs utilizing a 36-question survey regarding anatomy and function, risk factors, diagnosis and treatment options, and expectations. Secondarily, we evaluated the effect of an educational video and informational handout created by the authors to improve understanding. Participants ≥ 18 years were recruited from the senior author’s clinic and online discussion platforms over a 5-month period; (3) Results: Baseline surveys were completed by 382 individuals: 56% men, 64% Caucasian, 27% with at least a master’s degree, and 56% with very little or no RCT knowledge. Mean correct answer scores improved from 47% to 68% posteducational intervention (p < 0.001). Males, higher education level, healthcare experience, and a higher self-rated understanding of RCTs were significantly correlated with higher survey performance (p < 0.001); (4) Conclusions: The public’s knowledge of RCTs at baseline was poor, with demographic factors correlating with survey performance. The educational intervention effectively enhanced participants’ understanding. By focusing on common misconceptions, this data can help clinicians align patient expectations and enhance patient outcomes.
Full article
(This article belongs to the Special Issue Shoulder Disorders: Diagnosis and Treatment)
►▼
Show Figures
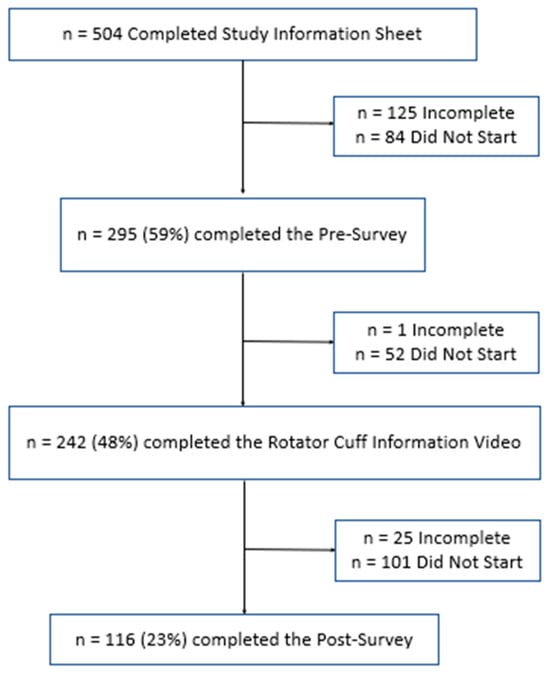
Figure 1
Open AccessArticle
Oral Habits in Childhood and Occlusal Pathologies: A Cohort Study
by
Mario Santos Barrera, David Ribas-Perez, Carolina Caleza Jimenez, Olga Cortes Lillo and Asunción Mendoza-Mendoza
Clin. Pract. 2024, 14(3), 718-728; https://doi.org/10.3390/clinpract14030057 - 24 Apr 2024
Abstract
►▼
Show Figures
Purpose: To analyse the relationship between the different habits that occur in childhood and the different malocclusions in the three planes of space. Material and methods: A clinical examination of 106 children between 5 and 12 years of age was carried out and
[...] Read more.
Purpose: To analyse the relationship between the different habits that occur in childhood and the different malocclusions in the three planes of space. Material and methods: A clinical examination of 106 children between 5 and 12 years of age was carried out and a survey validated by professors of the Faculty of Dentistry of the University of Seville was made for the parents in order to identify the habits and relate them to the possible malocclusions detected in the child’s mouth. Results: 72.64% of the sample presented a malocclusion in at least one of the three planes of space, with a similar distribution. When correlating the variables, statistically significant relationships were observed in the vertical plane with atypical swallowing (p = 0 < 0.05; V > 0.3) and lip sucking (p = 0 < 0.05; V > 0.3) and in the horizontal plane with oral breathing (p = 0 < 0.05; V > 0.3), atypical swallowing (p = 0 < 0.05; V < 0.3) and digital sucking (p = 0 < 0.05; V < 0.3). Conclusions: It has been observed that the prevalence and prolongation of habits in childhood is increasing, so it is essential to detect pernicious habits at an early age to prevent the establishment of malocclusions and to favour the correct craniofacial growth of the child.
Full article
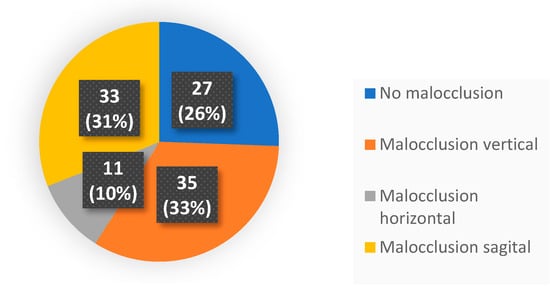
Figure 1
Open AccessArticle
The Contribution of Genetic Testing in Optimizing Therapy for Patients with Recurrent Depressive Disorder
by
Rita Ioana Platona, Florica Voiță-Mekeres, Cristina Tudoran, Mariana Tudoran and Virgil Radu Enătescu
Clin. Pract. 2024, 14(3), 703-717; https://doi.org/10.3390/clinpract14030056 - 23 Apr 2024
Abstract
►▼
Show Figures
(1) Background: The aim of this study was to analyze the impact of pharmacogenetic-guided antidepressant therapy on the 12-month evolution of the intensity of depressive symptoms in patients with recurrent depressive disorder (RDD) in comparison to a control group of depressive subjects who
[...] Read more.
(1) Background: The aim of this study was to analyze the impact of pharmacogenetic-guided antidepressant therapy on the 12-month evolution of the intensity of depressive symptoms in patients with recurrent depressive disorder (RDD) in comparison to a control group of depressive subjects who were treated conventionally. (2) Methods: This prospective longitudinal study was conducted between 2019 and 2022, and the patients were evaluated by employing the Hamilton Depression Rating Scale (HAM-D), Hamilton Anxiety Rating Scale (HAM-A) and the Clinical Global Impressions Scale: Severity and Improvement. We followed them up at 1, 3, 6, and 12 months. (3) Results: Of the 76 patients with RDD, 37 were tested genetically (Group A) and 39 were not (Group B). Although the patients from Group A had statistically significantly more severe MDD at baseline than those from Group B (p < 0.001), by adjusting their therapy according to the genetic testing, they had a progressive and more substantial reduction in the severity of RDD symptoms [F = 74.334; η2 = 0.674; p < 0.001], indicating a substantial association with the results provided by the genetic testing (67.4%). (4) Conclusions: In patients with RDD and a poor response to antidepressant therapy, pharmacogenetic testing allows for treatment adjustment, resulting in a constant and superior reduction in the intensity of depression and anxiety symptoms.
Full article
Figure 1
Open AccessArticle
Blood Count and Renal Functionality Assessments in the Emergency Section Disclose Morbidity and Mortality in Omicron COVID-19 Patients: A Retrospective Study
by
Eqrem Rusi, Fiorenza Pennacchia, Wael Abu Ruqa, Maria Antonella Zingaropoli, Patrizia Pasculli, Giuseppina Talarico, Giuseppe Bruno, Christian Barbato, Antonio Minni, Luigi Tarani, Gioacchino Galardo, Francesco Pugliese, Marco Lucarelli, Maria Rosa Ciardi, Luigi Meucci, Giampiero Ferraguti and Marco Fiore
Clin. Pract. 2024, 14(3), 685-702; https://doi.org/10.3390/clinpract14030055 - 23 Apr 2024
Abstract
Background: SARS-CoV-2 is the coronavirus responsible for the COVID-19 pandemic. Even though we are no longer in a pandemic situation, people are still getting infected, some of them need hospitalization and a few of them die. Methods: We conducted a retrospective
[...] Read more.
Background: SARS-CoV-2 is the coronavirus responsible for the COVID-19 pandemic. Even though we are no longer in a pandemic situation, people are still getting infected, some of them need hospitalization and a few of them die. Methods: We conducted a retrospective study including 445 patients who accessed the Emergency Section of Policlinico Umberto I, Rome, Italy, where they had routine blood exams. In this study, we focused on the complete blood count, serum creatinine and azotemia. The data were analyzed using ANOVA, Spearman correlation and ROC analyses. They were divided into four groups based on their clinical outcomes: (1) the emergency group (patients who had mild forms and were quickly discharged); (2) the hospital ward group (patients who were admitted to the emergency section and were then hospitalized in a COVID-19 ward); (3) the intensive care unit (ICU) group (patients who required intensive assistance after the admission in the emergency section); (4) the deceased group (patients who had a fatal outcome after admission to the emergency section). Results: We found significant changes for creatinine, azotemia, hematocrit, mean corpuscular hemoglobin concentration, basophils, monocytes, red blood cell distribution width, hemoglobin, hematocrit and red blood cell numbers using ANOVA according to their clinical outcomes, particularly for the deceased group. Also, we found linear correlations of clinical outcomes with eosinophils, hemoglobin, hematocrit, mean corpuscular hemoglobin concentration, lymphocyte, neutrophil, platelet and red blood cell number and red blood cell distribution width. Conclusions: This study discloses an early association between “classical” routine blood biomarkers and the severity of clinical outcomes in Omicron patients.
Full article
(This article belongs to the Topic Multifaceted Efforts from Basic Research to Clinical Practice in Controlling COVID-19 Disease)
►▼
Show Figures
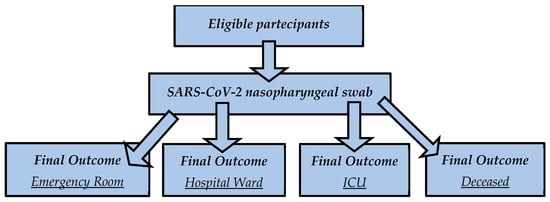
Figure 1
Open AccessArticle
A Worldwide Bibliometric Analysis of Published Literature Assessing Fear of COVID-19
by
Jesús Cebrino and Silvia Portero de la Cruz
Clin. Pract. 2024, 14(3), 672-684; https://doi.org/10.3390/clinpract14030054 - 23 Apr 2024
Abstract
Many people experience intense fear of COVID-19. The purpose of this study was to provide a comprehensive visual overview of the published literature from 2020 to 2022 assessing fear of COVID-19. From 2020 to 2022, we employed the Scopus database to conduct a
[...] Read more.
Many people experience intense fear of COVID-19. The purpose of this study was to provide a comprehensive visual overview of the published literature from 2020 to 2022 assessing fear of COVID-19. From 2020 to 2022, we employed the Scopus database to conduct a bibliometric analysis. We used the VOSviewer program to perform the author co-citation analysis, Mapchart to produce a worldwide map, and Wordart to make a word cloud image. From the 1769 records examined, 1654 (93.50%) were articles, with English being the most common language (96.31%). From 2020 to 2022, annual citations experienced significant growth (R2 = 99.91%; p = 0.0195). The Institut National de la Santé et de la Recherche Médicale (INSERM, France) and China led in terms of publication output (n = 36; n = 255). M. D. Griffiths authored the highest number of articles (n = 21). The most active journal was the International Journal of Environmental Research and Public Health (n = 146), and the most prevalent keyword was “human/s” (11.51%). This bibliometric analysis evaluates the quality of the research on fear of the pandemic and the crisis management of COVID-19, which can provide managers and researchers with crucial insights for future decision making.
Full article
(This article belongs to the Special Issue 2024 Feature Papers in Clinics and Practice)
►▼
Show Figures
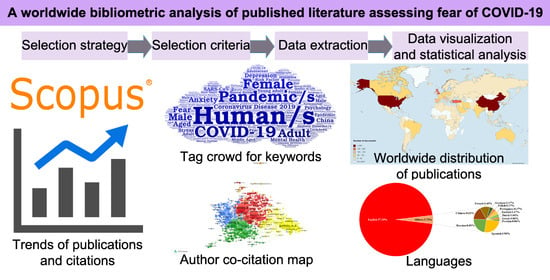
Graphical abstract
Open AccessArticle
Precise Prediction of Long-Term Urinary Incontinence after Robot-Assisted Laparoscopic Radical Prostatectomy by Readily Accessible “Everyday” Diagnostics during Post-Surgical Hospitalization
by
Mirjam Naomi Mohr, Hannah Maria Ploeger, Marianne Leitsmann, Conrad Leitsmann, Fabian Alexander Gayer, Lutz Trojan and Mathias Reichert
Clin. Pract. 2024, 14(3), 661-671; https://doi.org/10.3390/clinpract14030053 - 23 Apr 2024
Abstract
Aim and Objectives: We aimed to test the predictive value of readily accessible and easily performed post-surgical “bedside tests” on their validity of long-term urinary incontinence (UI) (≥12 months) in patients following robot-assisted laparoscopic radical prostatectomy (RALP). Material and Methods: Patients undergoing RALP
[...] Read more.
Aim and Objectives: We aimed to test the predictive value of readily accessible and easily performed post-surgical “bedside tests” on their validity of long-term urinary incontinence (UI) (≥12 months) in patients following robot-assisted laparoscopic radical prostatectomy (RALP). Material and Methods: Patients undergoing RALP between July 2020 and March 2021 were prospectively included and subdivided into two groups based on their pad usage after 12 months (0 vs. ≥1 pad). After catheter removal, patients performed a 1 h pad test, documented the need for pad change in a micturition protocol and received post-voiding residual urine volume ultrasound. Univariate and multivariable analyses were used to demonstrate the predictive value of easily accessible tests applied after catheter removal for UI following RALP. Results: Of 109 patients, 47 (43%) had to use at least one pad (vs. 62 (57%) zero pads) after 12 months. Univariate testing showed a significant difference in urine loss between both groups evaluated by the 1 h pad test performed within 24 h after catheter removal (70% < 10 mL, vs. 30% ≥ 10 mL, p = 0.004) and in the need for pad change within the first 24 h after catheter removal (14% dry pads vs. 86% wet pads, p = 0.003). In multivariable analyses, the combination of both tests (synoptical incontinence score) could be confirmed as an independent predictor for UI after 12 months (p = 0.011). Conclusions: Readily accessible “everyday” diagnostics (pad test/change of pads after catheter removal) following RALP seem to be associated with a higher rate of long-term UI. This finding is crucial since patients with a potentially higher need for patient education and counselling can be identified using these readily accessible tests. This could lead to a higher patient satisfaction and improved outcomes.
Full article
Open AccessReview
Concomitant Panniculectomy in Abdominal Wall Reconstruction: A Narrative Review Focusing on Obese Patients
by
Salvatore Giordano, Andre’ Salval and Carlo Maria Oranges
Clin. Pract. 2024, 14(2), 653-660; https://doi.org/10.3390/clinpract14020052 - 22 Apr 2024
Abstract
The global prevalence of obesity continues to rise, contributing to an increased frequency of abdominal wall reconstruction procedures, particularly ventral hernia repairs, in individuals with elevated body mass indexes. Undertaking these operations in obese patients poses inherent challenges. This review focuses on the
[...] Read more.
The global prevalence of obesity continues to rise, contributing to an increased frequency of abdominal wall reconstruction procedures, particularly ventral hernia repairs, in individuals with elevated body mass indexes. Undertaking these operations in obese patients poses inherent challenges. This review focuses on the current literature in this area, with special attention to the impact of concomitant panniculectomy. Obese individuals undergoing abdominal wall reconstruction face elevated rates of wound healing complications and hernia recurrence. The inclusion of concurrent panniculectomy heightens the risk of surgical site occurrences but does not significantly influence hernia recurrence rates. While this combined approach can be executed in obese patients, caution is warranted, due to the higher risk of complications. Physicians should carefully balance and communicate the potential risks, especially regarding the increased likelihood of wound healing complications. Acknowledging these factors is crucial in shared decision making and ensuring optimal patient outcomes in the context of abdominal wall reconstruction and related procedures in the obese population.
Full article
(This article belongs to the Special Issue 2024 Feature Papers in Clinics and Practice)
►▼
Show Figures
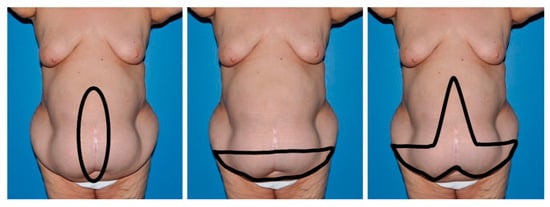
Figure 1
Open AccessArticle
A Phase II Study of Neoadjuvant Chemoradiotherapy with Docetaxel, Cisplatin and 5-FU Followed by Surgical Resection in the Treatment of Locally Advanced Esophagogastric Junction Cancer and Locally Advanced Esophageal Cancer
by
Chien-Chih Chen, Hui-Ling Yeh, Cheng-Yeh Chuang and Chung-Ping Hsu
Clin. Pract. 2024, 14(2), 642-652; https://doi.org/10.3390/clinpract14020051 - 22 Apr 2024
Abstract
►▼
Show Figures
Purpose: We conducted a phase II study evaluating chemoradiotherapy in patients with advanced esophageal cancer, using the docetaxel, cisplatin, and 5-fluorouracil (DCF) regimen followed by surgery. The primary purposes of this clinical trial were to assess the efficacy and safety of chemoradiotherapy employing
[...] Read more.
Purpose: We conducted a phase II study evaluating chemoradiotherapy in patients with advanced esophageal cancer, using the docetaxel, cisplatin, and 5-fluorouracil (DCF) regimen followed by surgery. The primary purposes of this clinical trial were to assess the efficacy and safety of chemoradiotherapy employing the DCF regimen in the treatment of advanced esophageal cancer. Material and methods: We enrolled a total of 24 newly diagnosed esophageal cancer patients between April 2015 and November 2017 in this prospective study. The radiotherapy regimen consisted of a total dose of 45 Gy in 25 fractions. The chemotherapy protocol included docetaxel 35 mg/m2 for 1 h on day 1 and day 29, cisplatin 35 mg/m2 for 1 h on day 1 and day 29, and 5-FU 400 mg/m2 for 24 h on day 1–4 and day 29–32. The patients who accepted the re-staging exam should undergo surgery in 4–8 weeks after the completion of radiotherapy. The primary endpoints of this study were disease-free survival (DFS), overall survival (OS), and the evaluation of hematologic toxicity. Results: The study population had a median age of 55.5 years, ranging from 44 to 66, with over 90% of the patients being male. The 5-year DFS was 37.1%, and the 5-year OS was 48.7%. The pathologic complete response rate was 45.8% (11/24). The most common types of toxicity were leukopenia and thrombocytopenia. No grade 3 or greater hematologic toxicity was reported. Conclusions: The use of the DCF regimen in neoadjuvant chemoradiotherapy followed by surgery demonstrated tolerable toxicity and achieved acceptable DFS and OS outcomes.
Full article
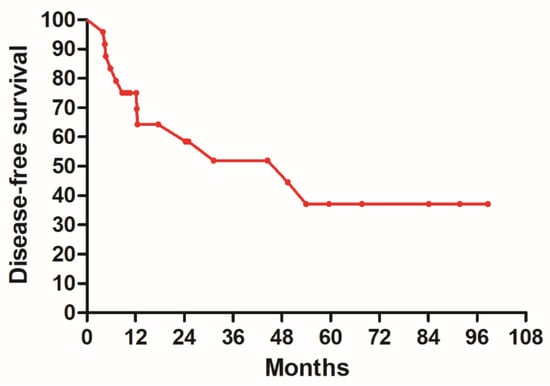
Figure 1
Open AccessCase Report
Clinical Approach to Patients with COVID-19 and Unrecognized Obstructive Sleep Apnea
by
Melany Ćurić, Frano Marinelli, Vuk Prica, Marijana Pavlović and Igor Barković
Clin. Pract. 2024, 14(2), 629-641; https://doi.org/10.3390/clinpract14020050 - 18 Apr 2024
Abstract
►▼
Show Figures
Purpose: We conducted a retrospective case series of seven male COVID-19 patients with respiratory failure and suspected OSA based on clinical features to evaluate the effects of undiagnosed obstructive sleep apnea (OSA) on COVID-19 outcomes and the response to a continuous positive airway
[...] Read more.
Purpose: We conducted a retrospective case series of seven male COVID-19 patients with respiratory failure and suspected OSA based on clinical features to evaluate the effects of undiagnosed obstructive sleep apnea (OSA) on COVID-19 outcomes and the response to a continuous positive airway pressure (CPAP) treatment. Cardiorespiratory polygraphy (CRP) and a continuous positive airway pressure treatment were used for diagnosis and management. They confirmed severe obstructive sleep apnea in all patients (apnea/hypopnea index > 30) and improved overnight oxygenation and symptoms at the 1-month follow-up. Conclusions: Undiagnosed obstructive sleep apnea may negatively impact COVID-19 outcomes by exacerbating respiratory failure. Recognition and treatment with continuous positive airway pressure can optimize the management of such patients.
Full article
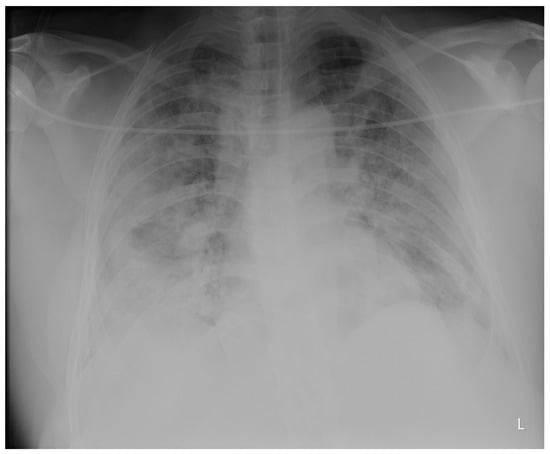
Figure 1
Open AccessCase Report
Successful Interventional Endovascular Management of Ruptured Penetrating Aortic Ulcer with Associated Enormous Right Pleural False Aneurysm
by
Andrei Emanuel Grigorescu, Andrei Anghel and Horea Feier
Clin. Pract. 2024, 14(2), 619-628; https://doi.org/10.3390/clinpract14020049 - 17 Apr 2024
Abstract
►▼
Show Figures
Penetrating aortic injuries represent critical medical emergencies that necessitate immediate intervention to prevent life-threatening consequences. When accompanied by the presence of an enormous right pleural false aneurysm, the clinical scenario becomes exceptionally rare and complex. This case report details the successful management of
[...] Read more.
Penetrating aortic injuries represent critical medical emergencies that necessitate immediate intervention to prevent life-threatening consequences. When accompanied by the presence of an enormous right pleural false aneurysm, the clinical scenario becomes exceptionally rare and complex. This case report details the successful management of a patient who presented with a penetrating aortic ulcer and an extensive false aneurysm within the right pleura, employing an interdisciplinary approach involving cardiac surgeons, cardiologists, interventional cardiologists, and radiologists. The pivotal intervention involved the deployment of a covered and bare stent graft into the descending thoracic aorta to seal the aortic rupture. The patient’s clinical condition stabilized postoperatively, with no signs of recurrent hemorrhage. This case underscores the importance of rapid diagnosis, timely intervention, and the collaborative efforts of a specialized medical team in successfully managing such complex vascular injuries. Early recognition and referral to specialized centers are essential for improving patient outcomes in cases of penetrating aortic injuries with associated giant pseudoaneurysms.
Full article
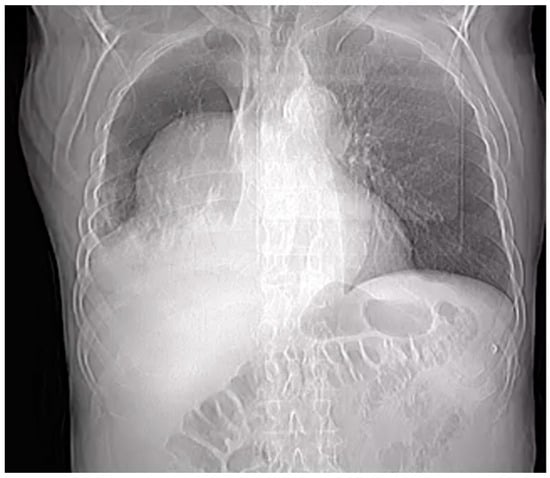
Figure 1
Open AccessOpinion
“Every Cloud Has a Silver Lining”: How Three Rare Diseases Defend Themselves from COVID-19 and What We Have Learnt from It
by
Martina Cacciapuoti, Ilaria Caputo, Lucia Federica Stefanelli, Paul A. Davis, Federico Nalesso and Lorenzo A. Calò
Clin. Pract. 2024, 14(2), 614-618; https://doi.org/10.3390/clinpract14020048 - 08 Apr 2024
Abstract
►▼
Show Figures
The process of SARS-CoV-2 infection, responsible for the COVID-19 pandemic, is carried out through different steps, with the interaction between ACE2 and Spike protein (S) being crucial. Besides of that, the acidic environment of endosomes seems to play a relevant role in the
[...] Read more.
The process of SARS-CoV-2 infection, responsible for the COVID-19 pandemic, is carried out through different steps, with the interaction between ACE2 and Spike protein (S) being crucial. Besides of that, the acidic environment of endosomes seems to play a relevant role in the virus uptake into cells and its intracellular replication. Patients affected by two rare genetic tubulopathies, Gitelman’s and Bartter’s Syndromes, and a rare genetic metabolic disease, Fabry Disease, have shown intrinsic protection from SARS-CoV-2 infection and COVID-19 on account of specific intrinsic features that interfere with the virus uptake into cells and its intracellular replication, which will be reported and discussed in this paper, providing interesting insights for present and future research.
Full article
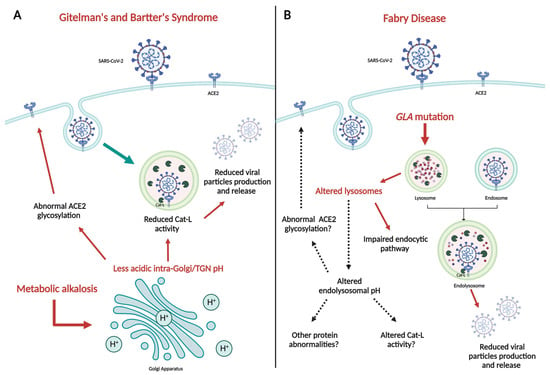
Figure 1
Open AccessArticle
Do MRI Results Represent Functional Outcomes Following Arthroscopic Repair of an Isolated Meniscus Tear in Young Patients?—A Prospective Comparative Cohort Study
by
Viktorija Brogaitė Martinkėnienė, Donatas Austys, Andrius Šaikus, Andrius Brazaitis, Giedrius Bernotavičius, Aleksas Makulavičius, Tomas Sveikata and Gilvydas Verkauskas
Clin. Pract. 2024, 14(2), 602-613; https://doi.org/10.3390/clinpract14020047 - 01 Apr 2024
Abstract
►▼
Show Figures
Background: The use of postoperative MRI to assess the healing status of repaired menisci is a long-standing issue. This study evaluates and compares functional and MRI outcomes following an arthroscopic meniscus repair procedure with the aim of postoperative MRI diagnostic accuracy clarification in
[...] Read more.
Background: The use of postoperative MRI to assess the healing status of repaired menisci is a long-standing issue. This study evaluates and compares functional and MRI outcomes following an arthroscopic meniscus repair procedure with the aim of postoperative MRI diagnostic accuracy clarification in young patients. Methods: A total of 35 patients under 18 years old who underwent isolated meniscus repair were included. The Pedi-IKDC score, Lysholm score, and Tegner activity index (TAS) were compared between the groups formed according to the Stroller and Crues three-grade classification of postoperative MRI-based evaluations. Grade 3 MRI views were classified as unhealed, grade 2 as partially healed, and grade 1 as fully healed within the repaired meniscus, whereas grade 3 cases were considered unsuccessful due to MRI evaluation. Results: MRI assessment revealed 4 cases of grade 1 (11.4%), 14 cases of grade 2 (40.8%), and 17 cases of grade 3 (48.0%) lesions. Pedi-IKDC and TAS scores were significantly higher among MRI grade 2 patients than among MRI grade 3 patients (p < 0.05). Weak negative correlations between MRI grades and all functional scales were found (p < 0.05). ROC analysis showed that Pedi-IKDC and TAS scores could correctly classify 77% and 71% of MRI grade 3 patients, respectively. The optimal cut-off values to detect grade 3 patients were 88.74 for the Pedi-IKDC score and 4.5 for the TAS score. Conclusions: To conclude, established functional score cut-off values may help identify unhealed meniscus repair patients.
Full article
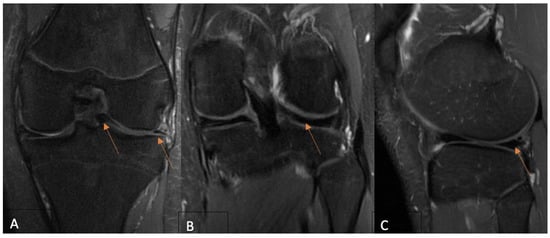
Figure 1
Open AccessArticle
Evaluating Global and Temporal Trends in Pancreas and Islet Cell Transplantation: Public Awareness and Engagement
by
Oscar A. Garcia Valencia, Charat Thongprayoon, Caroline C. Jadlowiec, Shennen A. Mao, Napat Leeaphorn, Pooja Budhiraja, Nadeen Khoury, Pradeep Vaitla, Supawadee Suppadungsuk and Wisit Cheungpasitporn
Clin. Pract. 2024, 14(2), 590-601; https://doi.org/10.3390/clinpract14020046 - 29 Mar 2024
Abstract
►▼
Show Figures
Background: Pancreas transplantation is a crucial surgical intervention for managing diabetes, but it faces challenges such as its invasive nature, stringent patient selection criteria, organ scarcity, and centralized expertise. Despite the steadily increasing number of pancreas transplants in the United States, there is
[...] Read more.
Background: Pancreas transplantation is a crucial surgical intervention for managing diabetes, but it faces challenges such as its invasive nature, stringent patient selection criteria, organ scarcity, and centralized expertise. Despite the steadily increasing number of pancreas transplants in the United States, there is a need to understand global trends in interest to increase awareness of and participation in pancreas and islet cell transplantation. Methods: We analyzed Google Search trends for “Pancreas Transplantation” and “Islet Cell Transplantation” from 2004 to 14 November 2023, assessing variations in search interest over time and across geographical locations. The Augmented Dickey–Fuller (ADF) test was used to determine the stationarity of the trends (p < 0.05). Results: Search interest for “Pancreas Transplantation” varied from its 2004 baseline, with a general decline in peak interest over time. The lowest interest was in December 2010, with a slight increase by November 2023. Ecuador, Kuwait, and Saudi Arabia showed the highest search interest. “Islet Cell Transplantation” had its lowest interest in December 2016 and a more pronounced decline over time, with Poland, China, and South Korea having the highest search volumes. In the U.S., “Pancreas Transplantation” ranked 4th in interest, while “Islet Cell Transplantation” ranked 11th. The ADF test confirmed the stationarity of the search trends for both procedures. Conclusions: “Pancreas Transplantation” and “Islet Cell Transplantation” showed initial peaks in search interest followed by a general downtrend. The stationary search trends suggest a lack of significant fluctuations or cyclical variations. These findings highlight the need for enhanced educational initiatives to increase the understanding and awareness of these critical transplant procedures among the public and professionals.
Full article
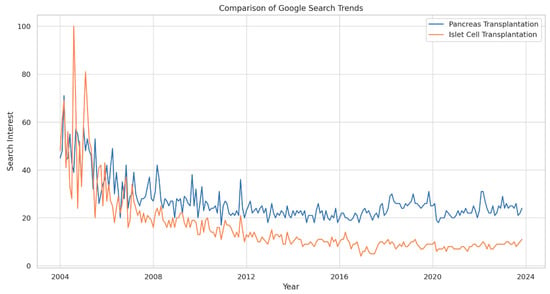
Figure 1
Open AccessArticle
Can Secondary Adhesive Capsulitis Complicate Calcific Tendinitis of the Rotator Cuff? An Ultrasound Imaging Analysis
by
Giovanni Tuè, Oriana Masuzzo, Francesco Tucci, Marco Cavallo, Anna Parmeggiani, Fabio Vita, Alberto Patti, Danilo Donati, Alessandro Marinelli, Marco Miceli and Paolo Spinnato
Clin. Pract. 2024, 14(2), 579-589; https://doi.org/10.3390/clinpract14020045 - 28 Mar 2024
Abstract
Background: Adhesive capsulitis (AC) of the glenohumeral joint is a recognized cause of pain associated with both active and passive restricted ranges of movement. AC can be subdivided into primary and secondary forms. Trauma, surgery, immobilization, and diabetes mellitus are the leading well-recognized
[...] Read more.
Background: Adhesive capsulitis (AC) of the glenohumeral joint is a recognized cause of pain associated with both active and passive restricted ranges of movement. AC can be subdivided into primary and secondary forms. Trauma, surgery, immobilization, and diabetes mellitus are the leading well-recognized causes of secondary AC. Calcific tendinitis/tendinitis (CT) of the rotator cuff is considered a possible trigger for AC, as reported in a few previous articles. However, there are no original investigations that assess the frequency and characteristics of this association. The aim of our research was to evaluate the presence of AC in a cohort of patients with a known CT condition of the rotator cuff by an ultrasound (US) examination. Materials and methods: We prospectively enrolled all the patients admitted at our single institution (October 2022–June 2023) for the preoperative US evaluation of a known CT condition. In these patients, we searched for parameters related to secondary AC. An axillary pouch (AP) thickness equal to or greater than 4 mm (or greater than 60% of the contralateral AP) was considered diagnostic of AC. Moreover, rotator interval (RI) thickness and the presence of effusion within the long-head biceps tendon (LHBT) sheath was also assessed in all patients. Results: A total of 78 patients (54F, 24M—mean age = 50.0 and range = 31–71 y.o.) were enrolled in the study. In 26 of those patients (26/78—33.3%), US signs of AC were detected. Notably, the mean AP thickness in patients with AC and CT was 3.96 ± 1.37 mm (Group 1) and 2.08 ± 0.40 mm in patients with CT only (Group 2). RI thickness was significantly greater in patients with superimposed AC: 2.54 ± 0.38 mm in Group 1 and 1.81 ± 0.41 mm in Group 2 (p < 0.00001). Moreover, effusion within the LHBT was significantly more frequently detected in patients with AC: 84.61% in Group 1 versus 15.79% in Group 2—p < 0.00001. Conclusion: US signs of AC are found in one-third of patients with CT of the rotator cuff, demonstrating that AC represents a frequent complication that should be routinely evaluated during US investigation to provide more personalized treatment strategies.
Full article
(This article belongs to the Special Issue Shoulder Disorders: Diagnosis and Treatment)
►▼
Show Figures
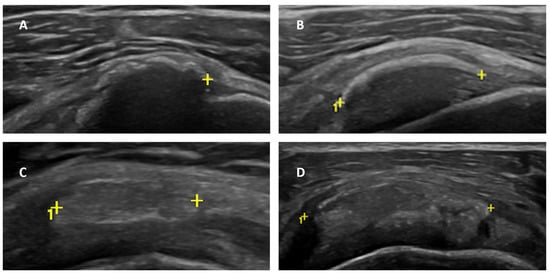
Figure 1
Open AccessArticle
Epidemiology of Hepatocellular Carcinoma in Taiwan
by
Yu-Wei Lai and Ching-Hu Chung
Clin. Pract. 2024, 14(2), 570-578; https://doi.org/10.3390/clinpract14020044 - 28 Mar 2024
Abstract
►▼
Show Figures
Background: Hepatocellular carcinoma (HCC) is a major contributor to the world’s cancer burden. Understanding the HCC incidence rate in Taiwan is thus an interesting avenue of research. Methods: From an NHI database, those patients who had been newly diagnosed with HCC and who
[...] Read more.
Background: Hepatocellular carcinoma (HCC) is a major contributor to the world’s cancer burden. Understanding the HCC incidence rate in Taiwan is thus an interesting avenue of research. Methods: From an NHI database, those patients who had been newly diagnosed with HCC and who had been listed on a registry in a catastrophic illness dataset during the years 2013–2021 were enrolled in this study. Antineoplastic agent usage and comorbidities were also studied. Results: The incidence rate of HCC decreased from 57.77 to 44.95 in 100,000 from 2013 to 2021. The average age of patients with HCC increased from 65.54 years old with a CCI score of 4.98 in 2013 to 67.92 years old with a CCI score of 5.49 in 2021. Among these HCC patients, the patients under antineoplastic agent treatment decreased from 53.47% to 31.41% from 2013 to 2021. The presence of comorbidities in HCC patients was about 55.77–83.01% with mild liver disease and 29.93–37.30% with diabetes (without complications) in the period 2013–2021. Conclusions: The incidence rate of HCC slightly decreased in Taiwan. Due to antineoplastic agent usage decreasing over time, these results may indicate that more early-stage HCC patients detected in recent years were mainly treated with surgeries.
Full article
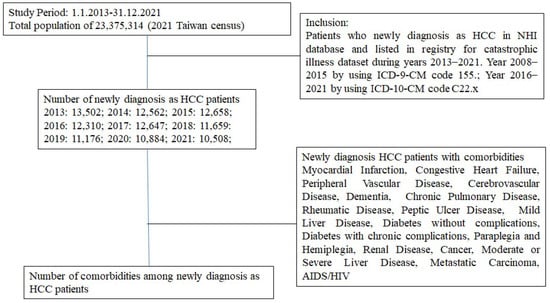
Figure 1
Open AccessArticle
Case Report of a Dental Implant with Conometric Abutment–Prosthetic Cap Connection: Advanced High-Resolution Imaging and Peri-Implant Connective Tissue Performance
by
Nicole Riberti, Emira D’Amico, Tania Vanessa Pierfelice, Michele Furlani, Alessandra Giuliani, Adriano Piattelli, Giovanna Iezzi and Luca Comuzzi
Clin. Pract. 2024, 14(2), 556-569; https://doi.org/10.3390/clinpract14020043 - 27 Mar 2024
Abstract
Background: In recent years, the use of conometric systems to connect dental implant abutments and prosthetic caps has been advocated because they seem to eliminate the side effects reported when using screw- and cement-connected prosthetic restorations. Objectives: The present case study
[...] Read more.
Background: In recent years, the use of conometric systems to connect dental implant abutments and prosthetic caps has been advocated because they seem to eliminate the side effects reported when using screw- and cement-connected prosthetic restorations. Objectives: The present case study is focused on conometric connection characterization and its performance in terms of the microarchitecture of peri-implant soft tissues by using a cross-linked approach based on optical microscopy and three-dimensional imaging. Methods: Two dental implants were characterized using micro-CT and another identical one was implanted into a patient; the latter was retrieved 45 days later due to changes in prosthetic needs. Afterward, the peri-implant soft tissues were investigated using synchrotron-based phase contrast imaging, histology, and polarized light microscopy. Results: Micro-CT analysis showed perfect adhesion between the abutment and prosthetic cap; histology and polarized light microscopy showed that connective tissue was richly present around the abutment retrieved from the patient. Moreover, the quantitative evaluation of connective tissues using synchrotron imaging, supported by artificial intelligence, revealed that this tissue was rich in mature collagen, with longitudinal and transverse collagen bundles intertwined. The number and connectivity of transverse bundles were consistently greater than those of the longitudinal bundles. Conclusion: It was found that the peri-implant soft tissue was already mature and well organized after only 45 days of implantation, supporting the hypothesis that conometric connections contribute to the significant stabilization of peri-implant soft tissues.
Full article
(This article belongs to the Special Issue New Trends, Materials, and Technologies and Consolidating Best Practices in Dentistry)
►▼
Show Figures
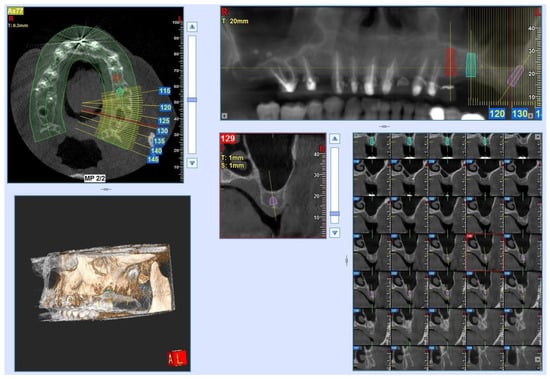
Figure 1
Open AccessArticle
The Influence of Wound Closure Techniques after Surgical Decompression in Patients with Carpal Tunnel Syndrome on Sleep Disturbance and Life Quality: A Prospective Comparison of Surgical Techniques
by
Veridijana Sunjic Roguljic, Luka Roguljic, Ivana Jukic and Vedran Kovacic
Clin. Pract. 2024, 14(2), 546-555; https://doi.org/10.3390/clinpract14020042 - 26 Mar 2024
Abstract
►▼
Show Figures
Background: The compression of the median nerve within the carpal tunnel is the cause of carpal tunnel syndrome (CTS). Surgical decompression is successful in improving sleep and quality of life, but the effect of tissue adhesives as a material for wound closure has
[...] Read more.
Background: The compression of the median nerve within the carpal tunnel is the cause of carpal tunnel syndrome (CTS). Surgical decompression is successful in improving sleep and quality of life, but the effect of tissue adhesives as a material for wound closure has not been investigated. The objective of the study was to evaluate sleep disorders and health-related life quality by comparing two methods for wound closure after carpal surgery in participants who were randomized to receive tissue adhesives or transcutaneous sutures. Methods: The subjects, aged 61.56 ± 12.03 years, were randomized to receive either tissue adhesives (n = 50) or suture-based wound closure (n = 50) using the Glubran Tiss 2® skin adhesive after subcutaneous running sutures. The outcomes were assessed during the 12-month postoperative follow-up. The Pittsburgh Sleep Quality Index (PQSI) and Insomnia Severity Scale (ISI) were used for the sleep disturbance assessment, and for the health-related quality of life assessment, the total SF-36 (36-Item Short Form Survey) was used. Results: The PQSI, ISI, and SF-36 were not statistically different between groups during the follow-up, except in the ISI score two weeks after surgery (9.40 ± 1.18 in the tissue adhesive group vs. 9.96 ± 1.09 in the suture-based group, p = 0.008). The PQSI, ISI, and SF-36 scores for all the subjects and groups were persistently improved at all the follow-up intervals after surgery. The total SF-36 score increased 12 months after surgery (49.84 ± 5.85 vs. 82.46 ± 5.68, p < 0.001). Conclusions: Cyanoacrylate-based adhesion material can be used for wound closure after open CTS decompression as a standard transcutaneous suture, and both techniques equally lead to improved sleep and life quality. The possible advantages of tissue adhesives include a faster reduction in the ISI.
Full article
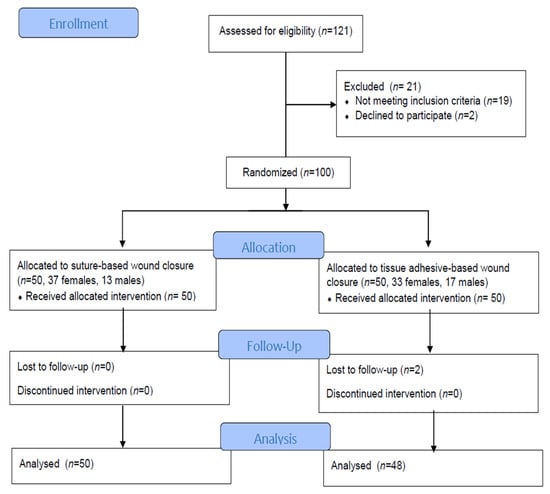
Figure 1
Open AccessArticle
Comparisons of the Rates of Large-for-Gestational-Age Newborns between Women with Diet-Controlled Gestational Diabetes Mellitus and Those with Non-Gestational Diabetes Mellitus
by
Sirida Pittyanont, Narongwat Suriya, Sirinart Sirilert and Theera Tongsong
Clin. Pract. 2024, 14(2), 536-545; https://doi.org/10.3390/clinpract14020041 - 25 Mar 2024
Abstract
(1) Objectives: The primary objective is to compare the rate of large-for-gestational-age (LGA) between women with diet-controlled gestational diabetes mellitus (GDM) and those with non-GDM, and to assess whether or not diet-controlled GDM is an independent factor of LGA fetuses. The secondary
[...] Read more.
(1) Objectives: The primary objective is to compare the rate of large-for-gestational-age (LGA) between women with diet-controlled gestational diabetes mellitus (GDM) and those with non-GDM, and to assess whether or not diet-controlled GDM is an independent factor of LGA fetuses. The secondary objectives are to compare the rates of other common adverse pregnancy outcomes, such as preeclampsia, cesarean section rate, preterm birth, and low Apgar score, between pregnancies with diet-controlled GDM and non-GDM pregnancies. (2) Methods: A retrospective cohort study was conducted on singleton pregnancies, diagnosed with GDM and non-GDM between 24 and 28 weeks of gestation, based on a two-step screening test. The prospective database of the obstetric department was accessed to retrieve the records meeting the inclusion criteria, and full medical records were comprehensively reviewed. The patients were categorized into two groups, GDM (study group) and non-GDM (control group). The main outcome was the rate of LGA newborns, and the secondary outcomes included pregnancy-induced hypertension, preterm birth, cesarean rate, low Apgar scores, etc. (3) Results: Of 1364 recruited women, 1342 met the inclusion criteria, including 1177 cases in the non-GDM group and 165 (12.3%) in the GDM group. Maternal age and pre-pregnancy BMI were significantly higher in the GDM group. The rates of LGA newborns, PIH, and cesarean section were significantly higher in the GDM group (15.1% vs. 7.1%, p-value < 0.001; 7.8% vs. 2.6%, p-value = 0.004; and 54.5% vs. 41.5%, p-value = 0.002; respectively). On logistic regression analysis, GDM was not significantly associated with LGA (odds ratio 1.64, 95% CI: 0.97–2.77), while BMI and gender were still significantly associated with LGA. Likewise, GDM was not significantly associated with the rate of PIH (odds ratio: 1.7, 95% CI: 0.825–3.504), while BMI and maternal age were significantly associated with PIH, after controlling confounding factors. (4) Conclusions: The rates of LGA newborns, PIH, and cesarean section are significantly higher in women with diet-controlled GDM than those with non-GDM. Nevertheless, the rates of LGA newborns and PIH are not directly caused by GDM but mainly caused high pre-pregnancy BMI and advanced maternal age, which are more commonly encountered among women with GDM.
Full article
(This article belongs to the Special Issue Clinical Nutrition in Metabolic Disorders)
Open AccessReview
Magnesium Is a Vital Ion in the Body—It Is Time to Consider Its Supplementation on a Routine Basis
by
Ákos Géza Pethő, Tibor Fülöp, Petronella Orosz and Mihály Tapolyai
Clin. Pract. 2024, 14(2), 521-535; https://doi.org/10.3390/clinpract14020040 - 22 Mar 2024
Abstract
►▼
Show Figures
The importance of maintaining proper magnesium intake and total body magnesium content in preserving human health remains underappreciated among medical professionals and laymen. This review aimed to show the importance of hypomagnesemia as a modifiable risk factor for developing disease processes. We searched
[...] Read more.
The importance of maintaining proper magnesium intake and total body magnesium content in preserving human health remains underappreciated among medical professionals and laymen. This review aimed to show the importance of hypomagnesemia as a modifiable risk factor for developing disease processes. We searched the PubMed database and Google Scholar using the keywords ‘magnesium’, ‘diabetes’, ‘cardiovascular disease’, ‘respiratory disease’, ‘immune system’, ‘inflammation’, ‘autoimmune disease’, ‘neurology’, ‘psychiatry’, ‘cognitive function’, ‘cancer’, and ‘vascular calcification’. In multiple contexts of the search terms, all reviews, animal experiments, and human observational data indicated that magnesium deficiency can lead to or contribute to developing many disease states. The conclusions of several in-depth reviews support our working hypothesis that magnesium and its supplementation are often undervalued and underutilized. Although much research has confirmed the importance of proper magnesium supply and tissue levels, simple and inexpensive magnesium supplementation has not yet been sufficiently recognized or promoted.
Full article
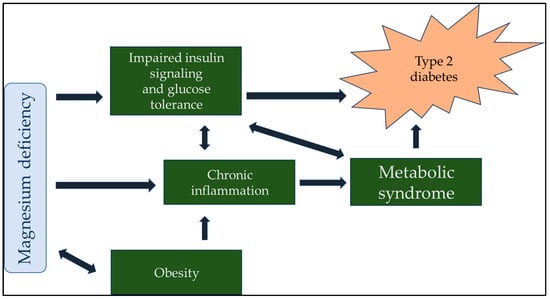
Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Clinics and Practice, JCM, JPM, Healthcare, Diagnostics
Pathology and Current State of Treatment of Chronic Pain
Topic Editors: Tatsunori Ikemoto, Young-Chang AraiDeadline: 31 July 2024
Topic in
Brain Sciences, Clinics and Practice, COVID, Life, Vaccines, Viruses
Multifaceted Efforts from Basic Research to Clinical Practice in Controlling COVID-19 Disease
Topic Editors: Yih-Horng Shiao, Rashi OjhaDeadline: 30 September 2024
Topic in
Biomedicines, Clinics and Practice, Diagnostics, JCM, Rheumato
Rheumatic Disorder: From Basic Science to Clinical Practice
Topic Editors: Giulia Cassone, Caterina Vacchi, Andreina ManfrediDeadline: 20 December 2024
Topic in
Healthcare, Pharmacy, Clinics and Practice, Nursing Reports, EJIHPE
Advancing the Knowledge and Application of Health Behavior Theories
Topic Editors: Yifei Liu, Dhananjay NayakankuppamDeadline: 31 December 2024

Conferences
Special Issues
Special Issue in
Clinics and Practice
Clinical Nutrition in Metabolic Disorders
Guest Editors: Charalampia Amerikanou, Aristea Gioxari, Efstathia PapadaDeadline: 15 May 2024
Special Issue in
Clinics and Practice
Clinical Practice of Artificial Intelligence in Diagnostic and Treatment Assistance
Guest Editors: Wisit Cheungpasitporn, Charat ThongprayoonDeadline: 31 May 2024
Special Issue in
Clinics and Practice
Management of Thyroid Diseases: Current Strategies and Future Directions
Guest Editor: Fausto FamàDeadline: 30 June 2024
Special Issue in
Clinics and Practice
Pediatric and Adolescent Psychiatry and Psychology: Current Treatments and Future Options
Guest Editor: Vassilis MartiadisDeadline: 31 July 2024